Ace Tips About How Does Electrode Potential Work
Electrode Potential Definition, Formula, Standard
Unlocking the Secrets of Electrode Potential
1. What's the Buzz About Electrode Potential?
Ever wondered how batteries power your phone or how corrosion eats away at metal? The secret lies, in part, with something called electrode potential. Now, that might sound intimidating, like something only a chemist could understand, but trust me, it's not as scary as it seems. Think of it as the electrical "personality" of a metal when it's dipped into a solution. It's all about how eager a metal atom is to either gain or lose electrons.
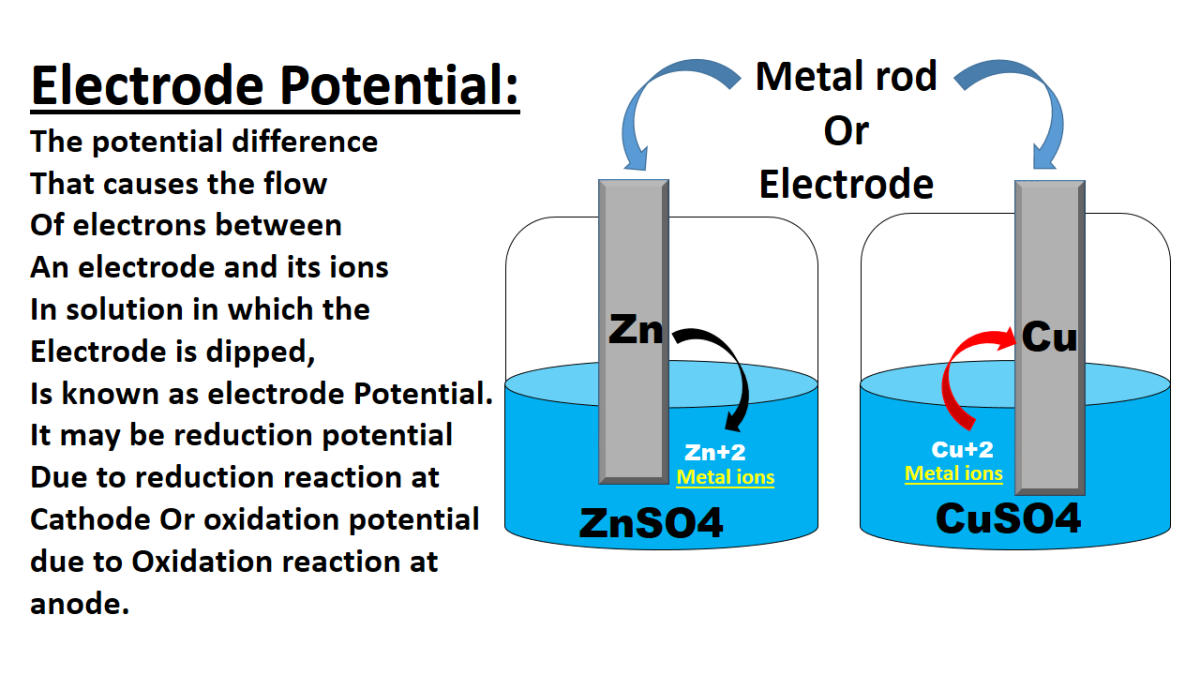
Essentially, electrode potential is a measure of the tendency of an electrode to lose or gain electrons when it is in equilibrium with a solution containing its ions. This tendency creates a potential difference between the electrode and the solution. It's like a tug-of-war, with the metal and the solution battling over electrons. The stronger the pull in one direction, the higher (or lower) the electrode potential.
And why is this important? Well, electrode potential dictates the direction of electron flow in a electrochemical cell, such as a battery. It explains why some metals corrode easily while others remain shiny and untarnished. So, understanding electrode potential provides you insights into a wide range of applications, from energy storage to corrosion protection.
Think of it like this: imagine you have a really charismatic person who can easily convince others to do what they want. That's like a metal with a high electrode potential — it's got a strong "desire" to gain electrons. On the other hand, a less persuasive person is like a metal with a low electrode potential — they're not as keen on attracting electrons. This "persuasiveness" directly translates to how things react when put together.

Standard Electrode Potentials Chemguide At Richard Reddish Blog
The Electron Tug-of-War
2. Getting Familiar with Redox Reactions
Electrode potential is all about something called redox reactions, short for oxidation-reduction reactions. These reactions are the heart and soul of electrochemistry. Oxidation, in this context, is when an atom loses electrons. Think of it as "giving away" electrons. Reduction, on the other hand, is when an atom gains electrons, essentially "receiving" them.
Now, here's the tricky part: oxidation and reduction always happen together. You can't have one without the other, like peanut butter and jelly. If one atom is losing electrons (oxidation), another atom must be gaining those electrons (reduction). It's a give-and-take relationship, a constant flow of electrons from one place to another.
Imagine a seesaw. On one side, you have oxidation, where the metal atom releases electrons and becomes a positively charged ion (a cation). On the other side, you have reduction, where another ion in the solution accepts these electrons, and can become a neutral atom or lower its positive charge. The electrode potential reflects the balance of this seesaw, indicating which process is more favorable under the given conditions. The higher the electrode potential, the greater the tendency for reduction to occur.
So, when you see a metal dissolving in acid, that's oxidation at work. The metal atoms are losing electrons and becoming ions in the solution. And what's being reduced? Usually, it's the hydrogen ions (H+) in the acid, which gain electrons to form hydrogen gas (H2). See? Oxidation and reduction, hand in hand, driving the whole process.

Standard Electrode Potential
3. Defining Standard Conditions
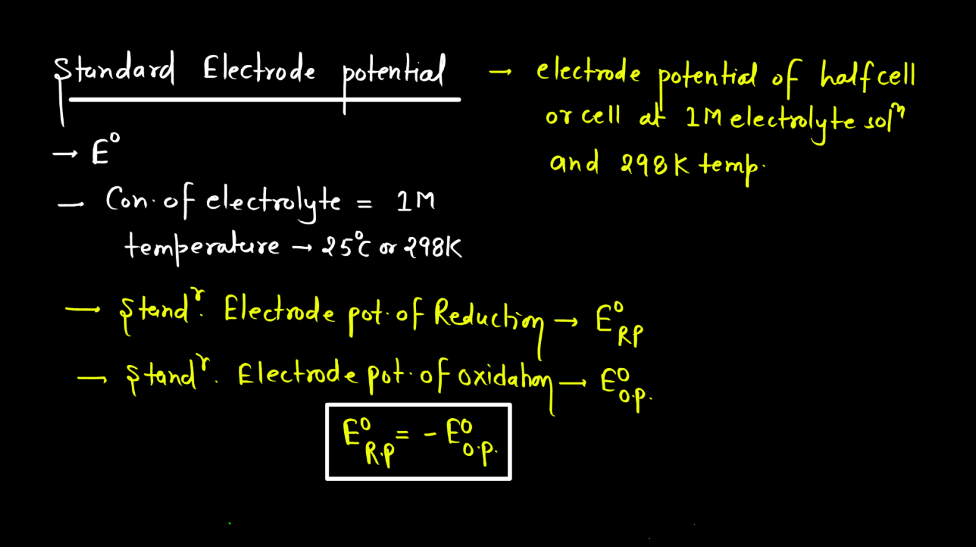
To compare the "electrical personalities" of different metals, scientists use a standard. This standard is called the standard electrode potential (E). It is the electrode potential measured under standard conditions: a temperature of 298 K (25C), a pressure of 1 atm (101.3 kPa), and a 1 M concentration of the metal ions in the solution.
Think of the standard electrode potential as a kind of "official" rating for each metal. It's like a standardized test for reactivity. The higher the standard electrode potential, the greater the tendency of the metal to be reduced (gain electrons) under these specific conditions. Metals with high positive values are considered noble metals (gold, platinum) as they resist oxidation (corrosion) and have a strong drive for reduction.
The standard electrode potential is always measured relative to a reference electrode — the standard hydrogen electrode (SHE). The SHE is assigned a potential of 0.00 V by definition, and all other electrode potentials are measured against it. It's like using a zero point on a ruler to measure the length of different objects. The SHE acts as a baseline, allowing us to compare the relative "eagerness" of other metals to gain electrons.
So, by comparing the standard electrode potentials of different metals, we can predict which metal will oxidize and which will reduce in a given electrochemical cell. This is incredibly useful for designing batteries, preventing corrosion, and understanding various chemical processes. The SHE, and its 0 voltage, provides the baseline measurement to determine reduction potential and the reactivity of a metal.

Electrode Potential Examples At Willis Beane Blog
Nernst Equation
4. Adjusting to Non-Standard Conditions
What happens when conditions aren't standard? What if the temperature isn't 25C, or the concentration of metal ions isn't 1 M? That's where the Nernst equation comes in. The Nernst equation adjusts the standard electrode potential to account for variations in temperature, and concentration. It's like a fine-tuning knob, allowing us to get a more accurate prediction of the electrode potential under real-world conditions.
The Nernst equation is a mathematical expression that relates the electrode potential (E) to the standard electrode potential (E), temperature (T), the number of electrons transferred (n), and the reaction quotient (Q). The reaction quotient reflects the relative amounts of reactants and products at any given time. Basically, it takes into account the specific conditions of the electrochemical cell.
So, if you increase the concentration of metal ions in the solution, the Nernst equation will tell you how the electrode potential changes. Similarly, if you raise the temperature, the equation will adjust the potential accordingly. It's a versatile tool that allows us to predict the behavior of electrochemical systems under a wide range of circumstances.
Think of it like a weather forecast. The standard electrode potential is like the average temperature for a particular day of the year. But the Nernst equation is like the actual temperature forecast, which takes into account factors like cloud cover, wind speed, and humidity to give you a more precise prediction. It's all about getting a better understanding of what's really happening in the system.
What Is Electrode Potential And Their Applications » Green Energy Material
Applications of Electrode Potential
5. Real-World Significance
Electrode potential isn't just some abstract concept confined to chemistry textbooks. It has numerous practical applications that impact our daily lives. Perhaps the most well-known application is in batteries. Batteries use electrochemical reactions involving metals with different electrode potentials to generate electricity.
Consider a simple zinc-copper battery. Zinc has a lower electrode potential than copper, so it readily oxidizes, releasing electrons. These electrons flow through an external circuit to the copper electrode, where they reduce copper ions in the solution. This flow of electrons creates an electric current that can power your devices. By carefully selecting metals with appropriate electrode potentials, we can design batteries with different voltages and energy capacities.
Another crucial application is in corrosion prevention. By understanding the electrode potentials of different metals, we can protect structures from corrosion. One common method is cathodic protection, where a more reactive metal (sacrificial anode) is connected to the metal structure to be protected. The sacrificial anode corrodes instead of the structure, effectively preventing corrosion. This is commonly used to protect pipelines, ships, and bridges.
Electrode potential also plays a role in electroplating, where a thin layer of metal is deposited onto a surface. By controlling the electrode potential, we can ensure a uniform and adherent coating. This technique is used to improve the appearance, durability, and corrosion resistance of various products. So, from powering your phone to protecting critical infrastructure, electrode potential is a fundamental concept that underpins many essential technologies.

9.4 Standard Electrode Potentials Chemistry LibreTexts
FAQs About Electrode Potential
6. Your Burning Questions Answered
Okay, let's tackle some common questions about electrode potential. I've tried to anticipate what's been swirling around in your brain!
Q: What's the difference between electrode potential and cell potential?A: Electrode potential refers to the potential of a single electrode relative to a reference electrode (usually SHE). Cell potential, on the other hand, is the potential difference between two electrodes in an electrochemical cell. It's the driving force behind the flow of electrons in the cell.
Q: Is a higher electrode potential always better?A: It depends on what you're trying to achieve. A higher electrode potential means a greater tendency for reduction, which is good if you want to prevent corrosion or design a battery cathode. However, a lower electrode potential means a greater tendency for oxidation, which is good if you want to create a sacrificial anode for cathodic protection.
Q: Can electrode potential change over time?A: Yes, electrode potential can change over time due to changes in temperature, concentration, or the composition of the electrode or solution. This is why the Nernst equation is so important for making accurate predictions in real-world scenarios.
Q: How to calculate electrode potential?A: You can calculate the electrode potential using the Nernst Equation. E = E - (RT/nF) * ln(Q), where:E is the cell potential.E is the standard cell potential.R is the ideal gas constant (8.314 J/molK).T is the temperature in Kelvin.n is the number of moles of electrons transferred in the balanced equation.F is Faraday's constant (approximately 96485 C/mol).Q is the reaction quotient, which is a measure of the relative amounts of products and reactants present in a reaction at a given time.
Hopefully, that clears things up a bit. Electrode potential might seem complex at first, but it's a fascinating concept with far-reaching implications.